Outstanding Info About How To Draw Resonance Structures

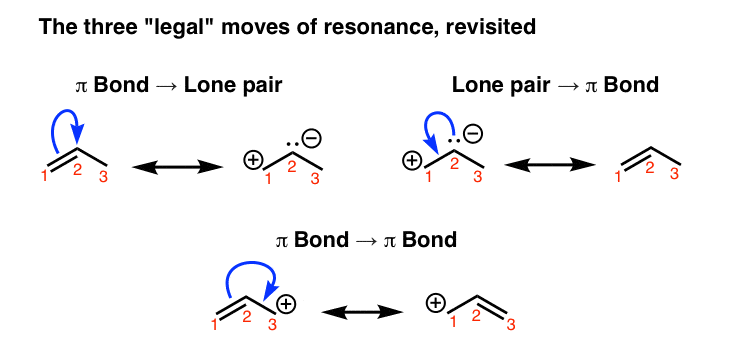
Lone pair electrons forms a π bond.
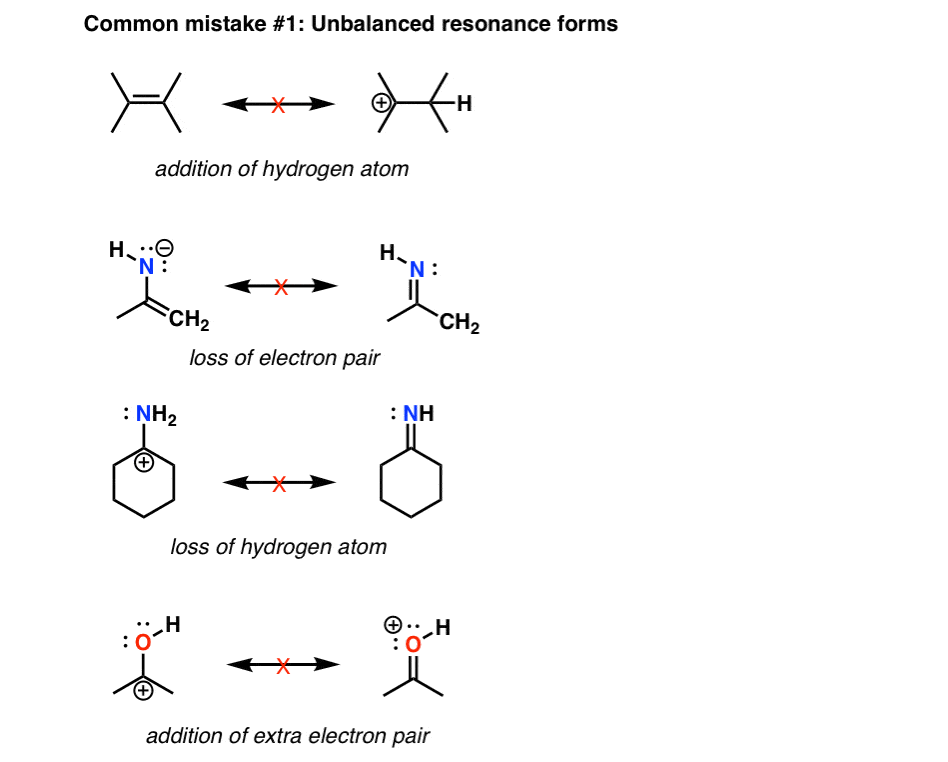
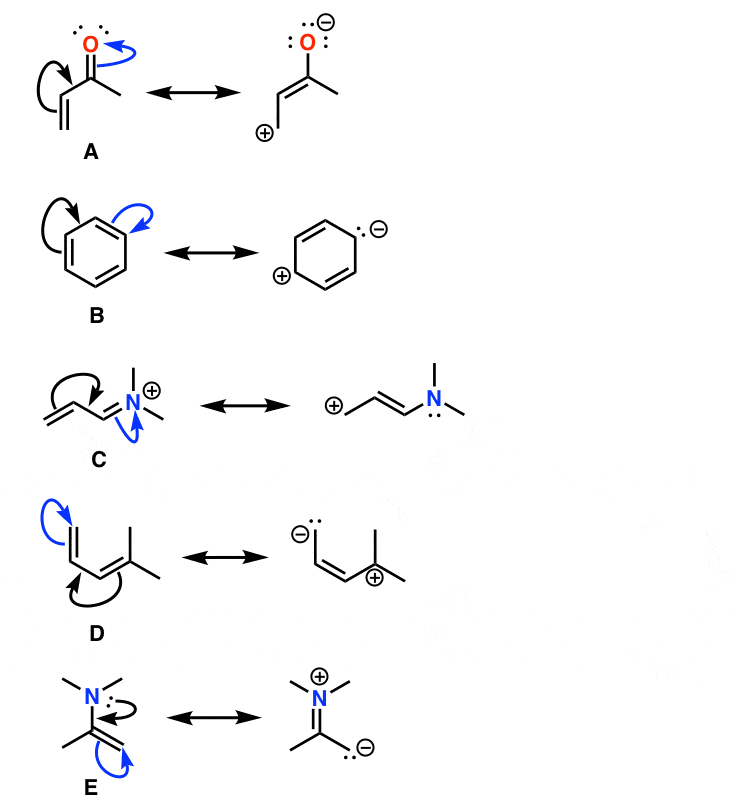
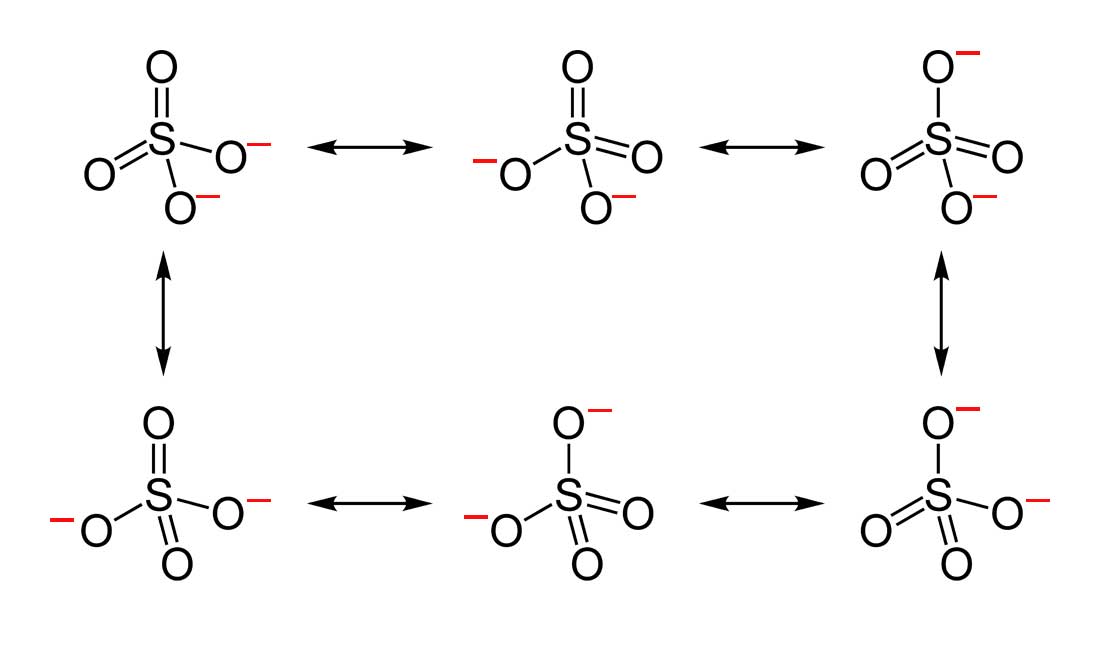
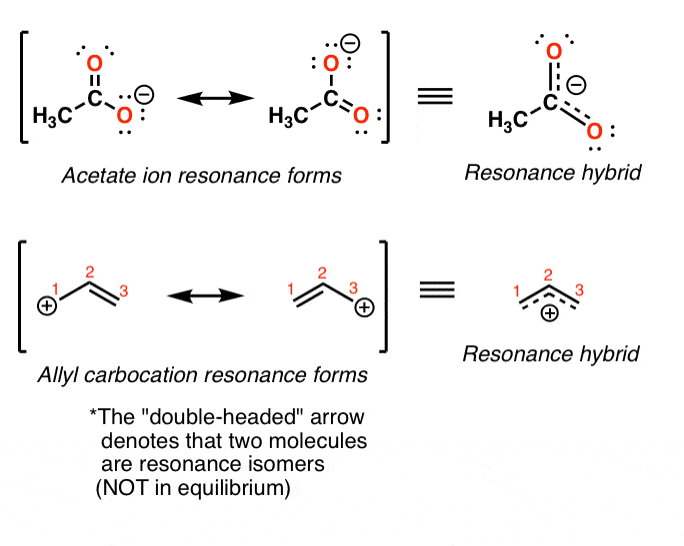
How to draw resonance structures. It explains how to identify the major resonance contr. Drawing significant resonance structures identify and use bonding patterns in molecules to draw acceptable resonance structures check structures to ensure proper formal. Steps for drawing all resonance structures & the resonance hybrid for a given lewis structure step 1:
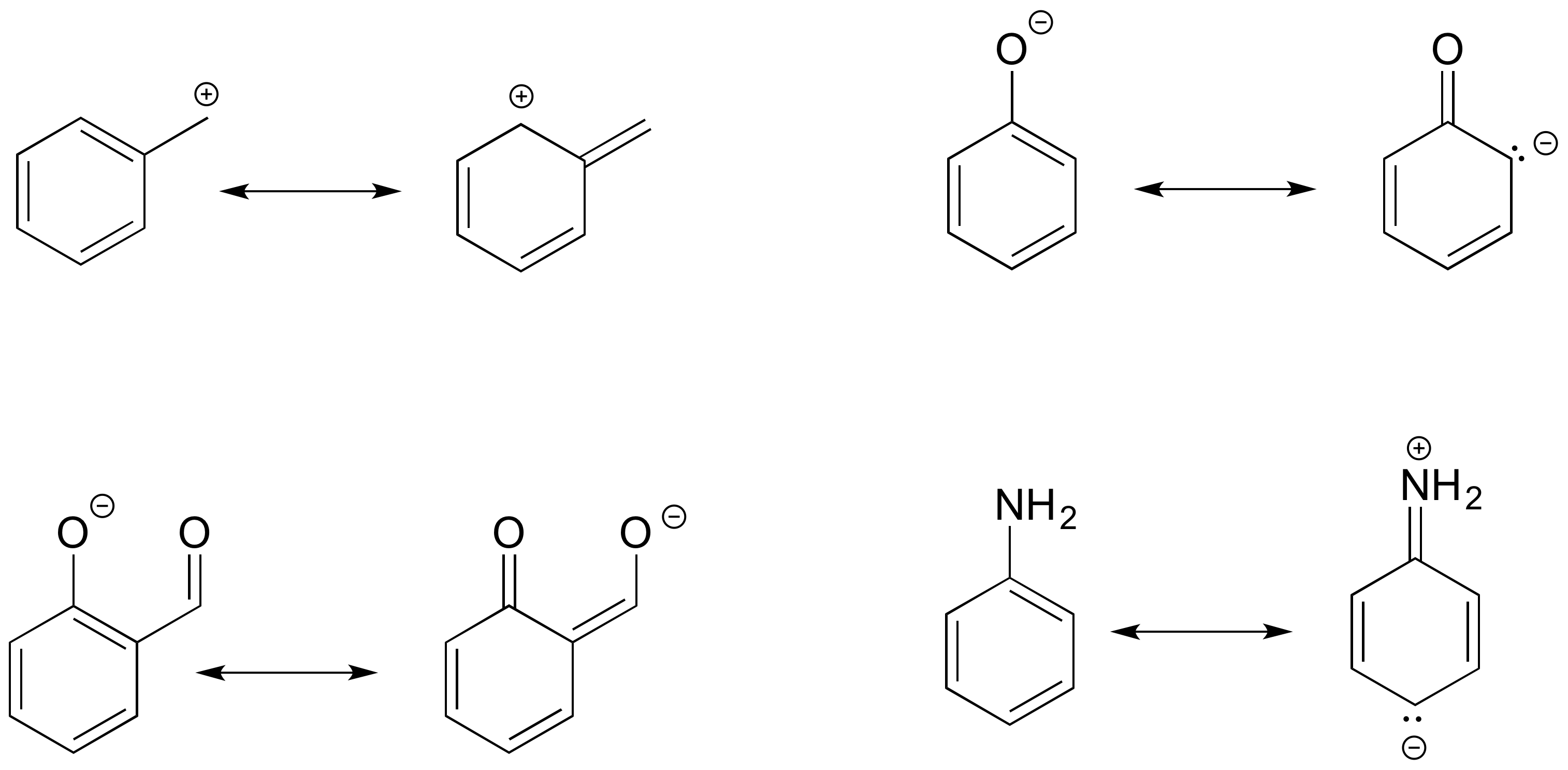
Drawing the resonance structure step 1: Draw the basic structure and look up each element in the periodic table. Answer keep it simple it is also possible to draw a resonance structure breaking a double bond.
Transfer the electrons on the oxygen with the negative charge and turn it into a double bond. Break the double bond between the positive. Draw a lewis structure from the molecular formula.
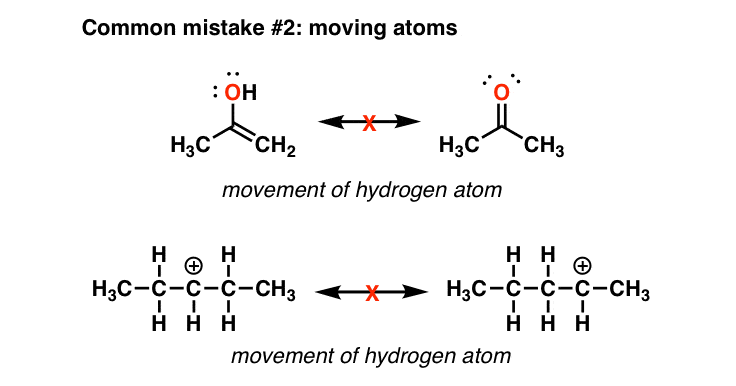
Delocalizing the electrons in a system with many #pi# electrons helps make that happen in molecules that we draw as resonance structures. To draw all resonance structures, take the lewis structure we drawn by using vespr rule. If so, move the double bonds around in a circle so that they are in the alternate.
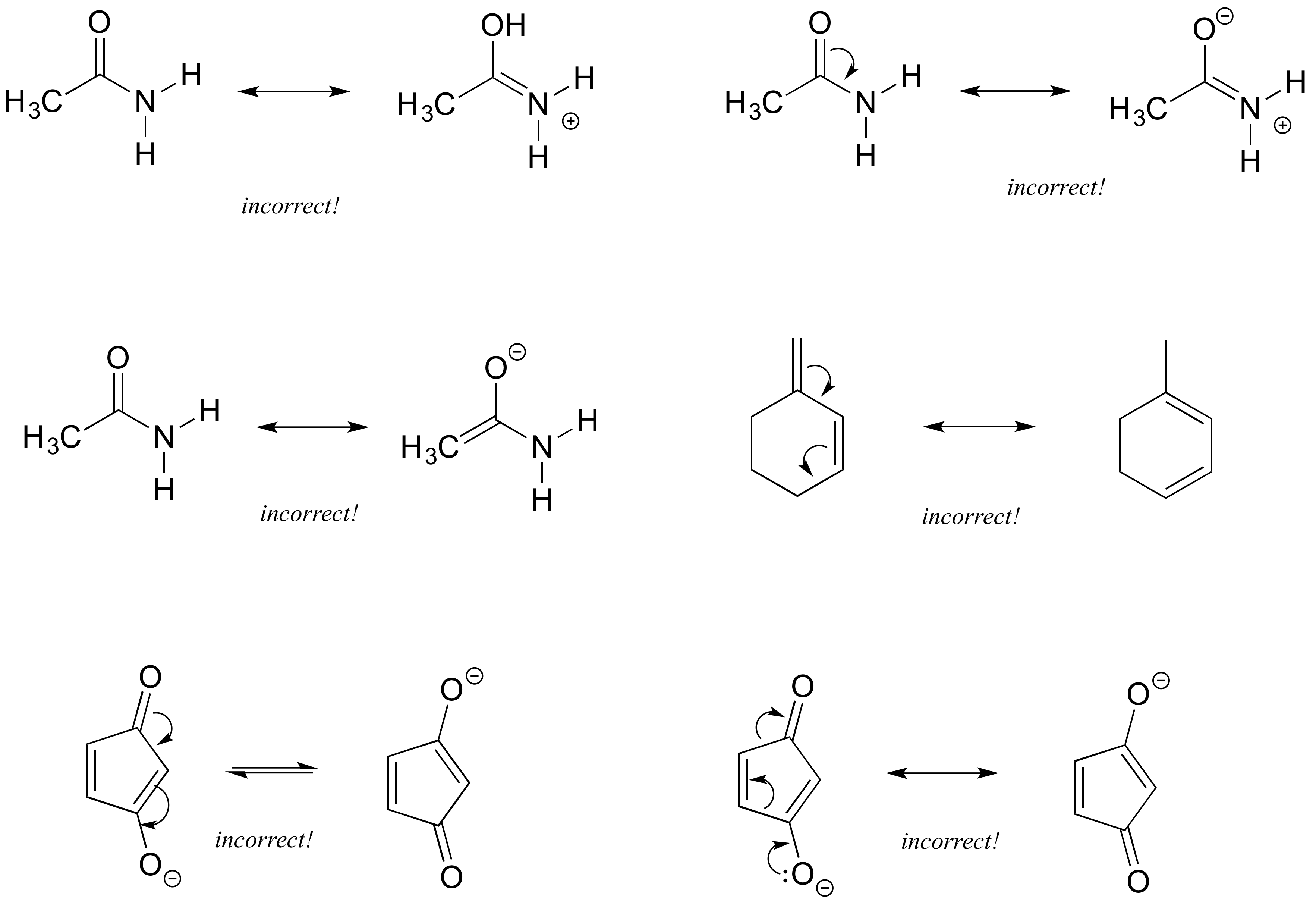
The “new” resonance structure should be a “product”. Use curved arrows to indicate the electron movement in the “original” resonance structure. Understand the rules of resonance and identify where electrons can flow to or away from.
This video explains the trick to draw resonance structures. The steps to draw resonance structures: If a resonance hybrid of this polyatomic ion is drawn from the set of lewis structures provided above, the partial.